
Membrane-Lipid Therapy
Definition
Clinical drugs that interact with membrane lipids and that modify the composition and structure of cell membranes can change the localization and/or activity of membrane proteins. Several drugs used to combat cancer, cardiovascular pathologies, obesity and other pathologies, regulate membrane lipid structure and they produce a concomitant alteration in the localization and activity of signaling proteins. The net result of these effects is the modulation of certain signaling pathways that reverse the pathological state.
Indeed, G proteins, protein kinase C (PKC) and heat shock proteins (HSPs) are among the proteins regulated by membrane-lipid therapy (Escribá et al., 1995; Escribá et al., 1997; Martínez et al., 2005; Torok et al., 2003) and the therapeutic agents used against cancer can inhibit cell proliferation or induce apoptosis and cell differentiation (Martínez et al., 2005; Martínez et al., 2005b). Regulation of membrane lipid composition and structure is also used to treat cardiovascular pathologies and obesity (Escribá et al., 2003; Alemany et al, 2004; Alemany et al., 2006).
Characteristics
Heterogeneity of membrane lipids and lipid structures.
Membranes are composed of thousands of different lipid molecules that interact dynamically to form the transient or stable structures used by many proteins as platforms for their activity and for their interactions with other proteins. Usually, integral (transmembrane) and peripheral (amphitropic) proteins show important specificities in their interactions with lipids. These preferences may be associated either with a defined type of membrane lipid or a given membrane lipid structure. Most proteins are sensitive to their lipid environment, so that their activity can be modified by changes in membrane lipid composition and structure. These changes in membrane lipid composition and structure may have a physiological basis or they may be a response to external stimuli. An example of the former is the change in cell membrane lipids in response to important changes in water temperature in cold-adapted fishes. Alternatively, one example of the latter is the variation observed in membrane lipids after the intake of a given substance (drug, food, toxin, etc.) (Escribá et al., 1995; Martínez et al., 2005).
Membrane lipids can organize into many more secondary structures than proteins (Fig 1) and nucleic acids in vitro. Moreover, the number of lipid species exceeds the number of different amino acids and nucleic acid bases by various orders of magnitude. In contrast with proteins and nucleic acids, the spatial relationship between the lipids that form membranes is not defined by covalent bonds, they usually move freely in this environment. Furthermore, since some structural aspects related to membranes still remain unclear, the behavior of lipids is less predictable. However, this does not mean that structure-function relationships are not established in membranes and that bulk thermodynamics can explain all physico-chemical properties of membranes. In fact, changes in the structure of lipids influence not only the physical behavior of membrane lipids (membrane structure) but also the activity of certain associated proteins (membrane function). In general, modifications in membrane lipid structure are reflected in changes in membrane lipid function. Thus, oleic acid (18:1 cisD9) induces a large increase in the non-lamellar (HII) phase propensity of membranes and it alters the interaction and activity of G proteins. In contrast, neither the trans analogue of oleic acid (elaidic acid) nor stearic acid (18:0) markedly influence the lamellar phase of membranes and accordingly, they do not influence G protein activity (Yang et al., 2005).
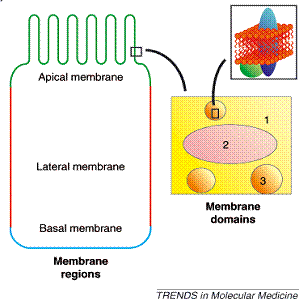
During recent years it has become accepted that the fluid mosaic model of membranes described more than 30 years ago by Singer and Nicolson (1972) is a somewhat simplified model which fails to take into account the presence of large membrane domains (e.g. the basal, lateral and apical membrane regions of polarized cells, such as glandular, endothelial and epithelial cells), as wall as the smaller yet highly abundant membrane structures (Fig 1: lipid rafts, synaptosomes, caveolae, coated pits, receptor clusters, etc.) (Escribá, 2006). These domains and structures are characterized by their characteristic lipid and protein composition. In this sense, while the membrane lipid composition most likely defines the presence of specific proteins, proteins may also influence the lipid composition of these domains.

Figure 1. Membrane lipid structure. Upper panel: Some examples of lipid shapes and their influence on membrane structure. Lipids with a small polar head (blue), such as phosphatidylethanolamine, have a molecular shape that resembles a truncated cone. They induce a negative curvature strain and favor the organization of membranes into inverted micelles (HII phases) or cubic (bicontinuous) structures. Lipids with a bulky polar head and only one acyl chain (e.g. lysophospholipids, green) have a molecular shape similar to an inverted cone and induce a positive curvature strain in membranes. They favor the formation of tubular (HI) or spheric micelles. Lipids such as phosphatidylcholine have similar cross-sectional areas for the polar head and hydrophobic region and resemble cylinders (red). They form lamellar phases, with no curvature strain. Lipid bilayers can form a number of different structures, some of them depicted here. Lower panel: In many polarized cells (e.g., small-intestine endothelial cells) there are specialized (e.g. apical [green], lateral [red] and basal [blue]) regions containing specific lipids and proteins (left). In a given membrane area (1), GPCR-cluster domains (2) and lipid-raft domains (3) can be well differentiated (right)
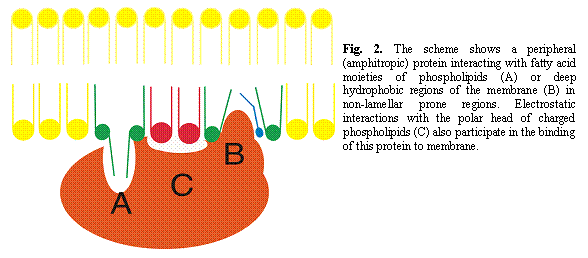
Development of anticancer “membrane-lipid therapy” drugs
It was proposed that the cytotoxic effects of anthracyclines on cancer cells may be exclusively exerted through their interaction with membranes (Tritton and Yee, 1982). Indeed, it was later demonstrated that they acted by regulating membrane lipid structure which altered the interaction between peripheral signaling proteins and the plasma membrane (Escribá et al., 1995). Subsequently, a potential anticancer drug under study, hexamethylene bisacetamide, was found to have a very similar effect on cell membranes, which was associated with the regulation of gene expression. In the knowledge that the mechanism of action was based on the regulation of membrane lipid structure, a number of lipid-interacting molecules were subsequently studied and accordingly, it was demonstrated that oleic acid and its derivatives are potent regulators of the membrane structure (Escribá et al., 1995; Vögler et al., 2004; Escribá, 2006; Yang et al., 2005). Subsequently, Minerval (the a-hydroxyl-derivative of oleic acid) was found to be a potent antitumor agent without displaying any relevant side effects.
Minerval is not the only drug used against cancer that interacts with membranes. As mentioned above, anthracyclines and hexamethylene bisacetamide also regulate membrane structure and peripheral protein-associated signaling. In addition, certain molecules that readily bind to membranes have been shown to have important anticancer effects. For instance, edelfosine [Et-18-OCH3 (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine)] and miltefosine [HePC (hexadecyl phosphocholine)] have an important hydrophobic moiety with long hydrocarbon chains (18 and 16 C atoms, respectively), and a polar region comprised of a phosphate group and a choline moiety (Fig. 3). This polar-apolar hybrid structure appears to be a common feature of these anticancer drugs that appear to be active in membrane-lipid therapy. This physico-chemical property of these drugs may allow them to interact with both the surface-interface of the membrane and with the hydrophobic core, facilitating more stable and long-lasting interactions, as well as inducing the relevant effects on membrane lipid structure. Thus, an interesting class of new anticancer drugs are those compounds known to bind to lipid molecules, such as the molecule NEO6002 (Fig 3). This drug results from the combination of gemcitabine with cardiolipin, a phospholipid typical of mitochondrial membranes, and it appears to be less toxic and more effective than gemcitabine alone (Chen et al., 2006). The lipid modification of gemcitabine induces the membrane-mediated internalization of the compound, which is not blocked by nucleoside transporter inhibitors. Another type of lipid-interacting compound is propofol-DHA (Fig 3), which combines a well-known anesthetic (propofol) with a polyunsaturated fatty acid (docosahexaenoic acid, DHA) that is present in membranes. The resulting compound has been shown to induce apoptosis in MDA-MB-231 breast cancer cells.
Most cell functions are localized in or around membranes, and lipids control the interaction and activity of many proteins. The relevance of lipids in the treatment of cancer is also highlighted by the lipid abnormalities identified in the membranes of patients with cancer. Thus, changes in the type or abundance of lipids and other types of membrane components may produce either positive or negative effects on health. Hence, membrane-lipid therapy is a new therapeutic approach that could be used in the treatment of cancer and other pathologies.
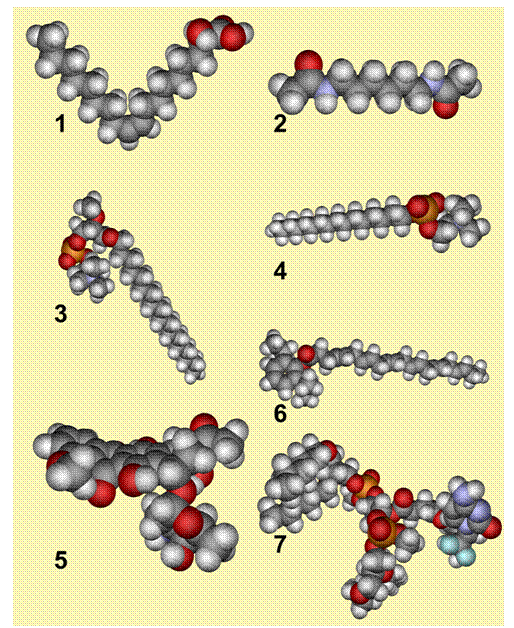
Fig. 3. Structures of the semi-empirical RHF calculations for minerval (1), HMBA (2), edelfosine (3), miltefosine (4), daunorubicin (5), propofol-DHA (6) and NEO6002 in gas phase. Carbon atoms are shown in grey, hydrogen in light grey, phosphorus in orange, oxygen in red, nitrogen in light blue and fluorine.
References
Alemany R, Terés S, Baamonde C, Benet M, Vögler O, Escribá PV (2004) 2-Hydroxyoleic acid: a new hypotensive molecule. Hypertension 43:249-253.
Alemany R, Vögler O, Terés S, Egea C, Baamonde C, Barceló F, Delgado C, Jakobs KH, Escribá PV (2006) Antihypertensive action of 2-hydroxyoleic acid in SHRs via modulation of the protein kinase A pathway and Rho Kinase. J. Lipid Res. 47:1762-1770.
Chen P, Chien PY, Khan AR, Sheikh S, Ali SM, Ahmad MU, Ahmad I (2006) In-vitro and in-vivo anti-cancer activity of a novel gemcitabine-cardiolipin conjugate. Anticancer drugs 17:53-61.
Crook T et al. (1992) Effects of phosphatidylserine in Alzheimer’s disease. Psychopharmacol. Bull. 28:61-66.
Escriba PV, Sastre M, García-Sevilla JA (1995) Disruption of cellular signaling pathways by daunomycin through destabilization of nonlamellar membrane structures. Proc. Natl. Acad. Sci. USA 92:7595-7599.
Escribá PV, Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, García-Sevilla JA (1997) Role of lipid polymorphism in G protein-membrane interactions: Nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc. Natl. Acad. Sci. USA 94:11375-11380.
Escribá PV, Sánchez-Domínguez JM, Alemany R, Perona JS, Ruiz-Gutierrez V (2003) Alteration of lipids, G proteins and PKC in cell membranes of elderly hypertensives. Hypertension 41:176-182.
Escribá PV (2006) Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol. Med. 12:34-43.
Martínez J, Vögler O, Casas J, Barceló F, Alemany R, Prades J, Nagy T, Baamonde C, Kasprzyk PG, Terés S, Saus C, Escribá PV (2005) Membrane structure modulation, protein kinase Ca activation, and anticancer activity of Minerval. Mol. Pharmacol. 67:531-540.
Martínez J, Gutiérrez A, Casas J, Lladó V, López-Bellan A, Besalduch J, Dopazo A, Escribá PV (2005b) The repression of E2F-1 is critical for the activity of Minerval against cancer. J. Pharmacol. Exp. Ther. 315:466-474.
Török Z, Tsvetkova NM, Balogh G, Horvath I, Nagy E, Penzes Z, Hargitai J, Bensaude O, Csermely P, Crowe JH, Maresca B, Vigh L (2003) Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc. Natl. Acad. Sci. USA 100:3131-3136.
Tritton TR, Yee G (1982) The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science 217:248-250.
Singer SJ, Nicolson GL (1972) The fluid mosaic model of cell membranes. Science 175:720-731.
Vögler O, Casas J, Capó D, Nagy T, Borchert G, Martorell G, Escribá PV (2004) The Gbg dimer drives the interaction of heterotrimeric G proteins with nonlamellar membrane structures. J. Biol. Chem. 279:36540-36545.
Willumeit R et al. (2005) Structural rearrangement of model membranes by the peptide antibiotic NK-2. Biochim. Biophys Acta 1669:125-134.
Yang Q, Alemany R, Casas J, Kitajka K, Lanier SM, Escribá PV (2005) Influence of the membrane lipid structure on signal processing via G protein-coupled receptors. Mol. Pharmacol. 68, 210-218.